- No Heat Affected Zone (HAZ)
- Fastest HAZ-Free laser machining process
- .00045” kerf width for nitinol implants
- .00025” kerf width for bioresorbable implants
- Validated laser manufacturing process
- 25+ years of nitinol stent manufacturing experience
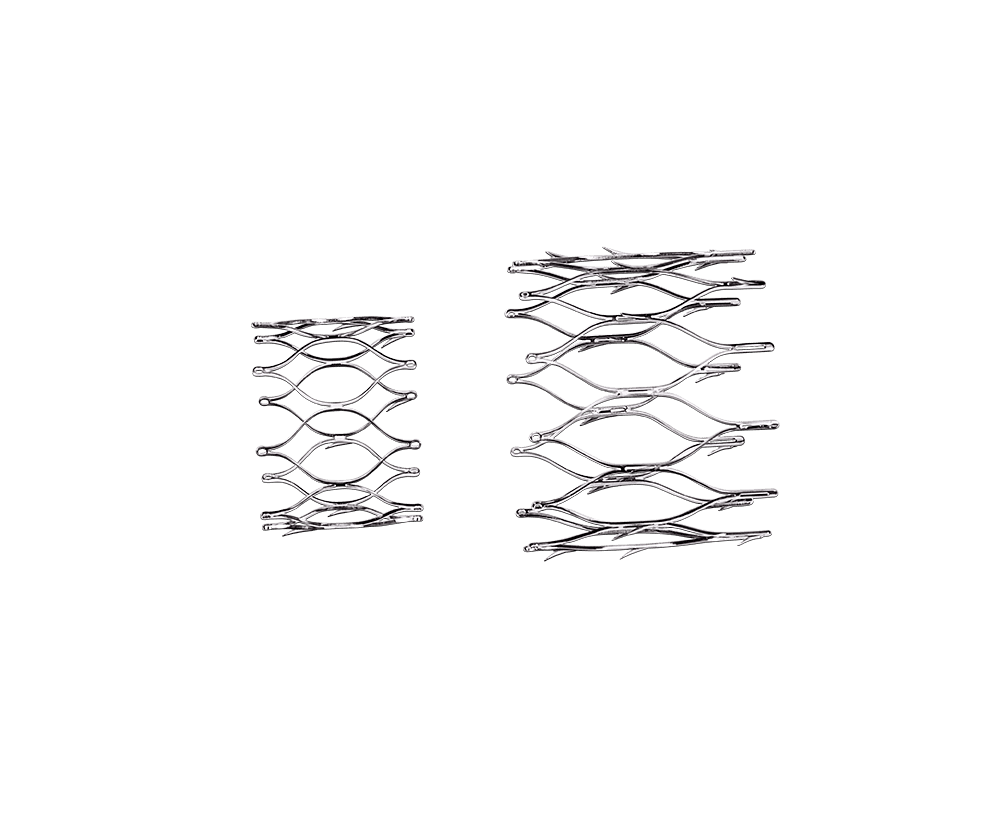
Norman Noble’s proprietary STEALTH athermal laser machining provides unmatched machining capabilities on a wide range of materials.
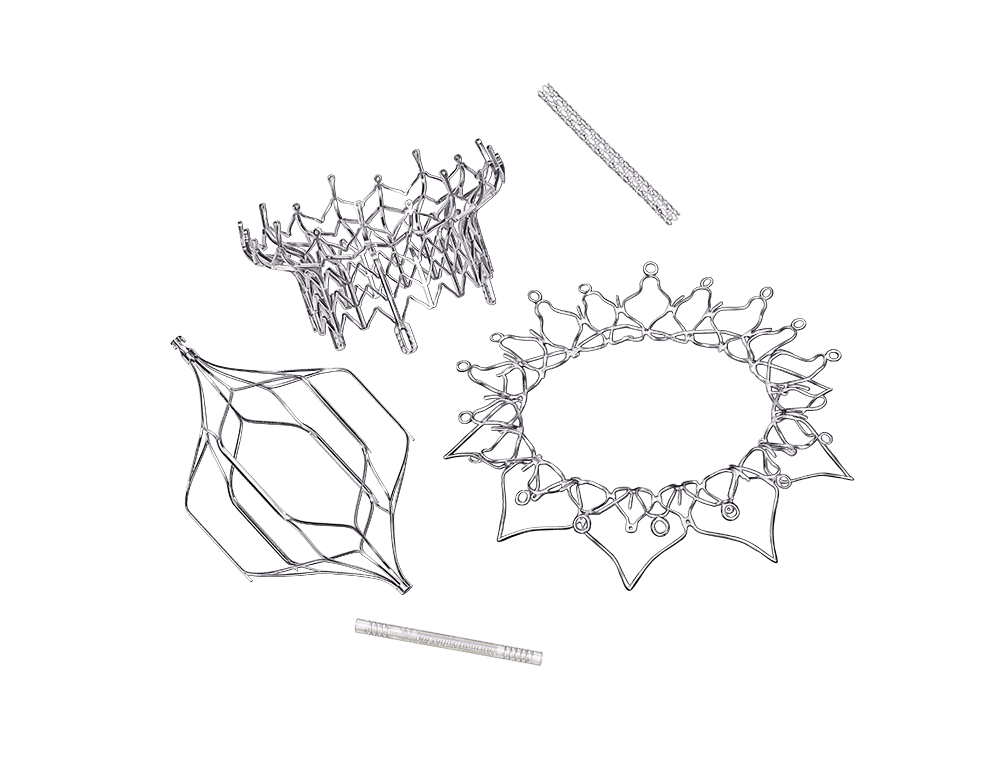
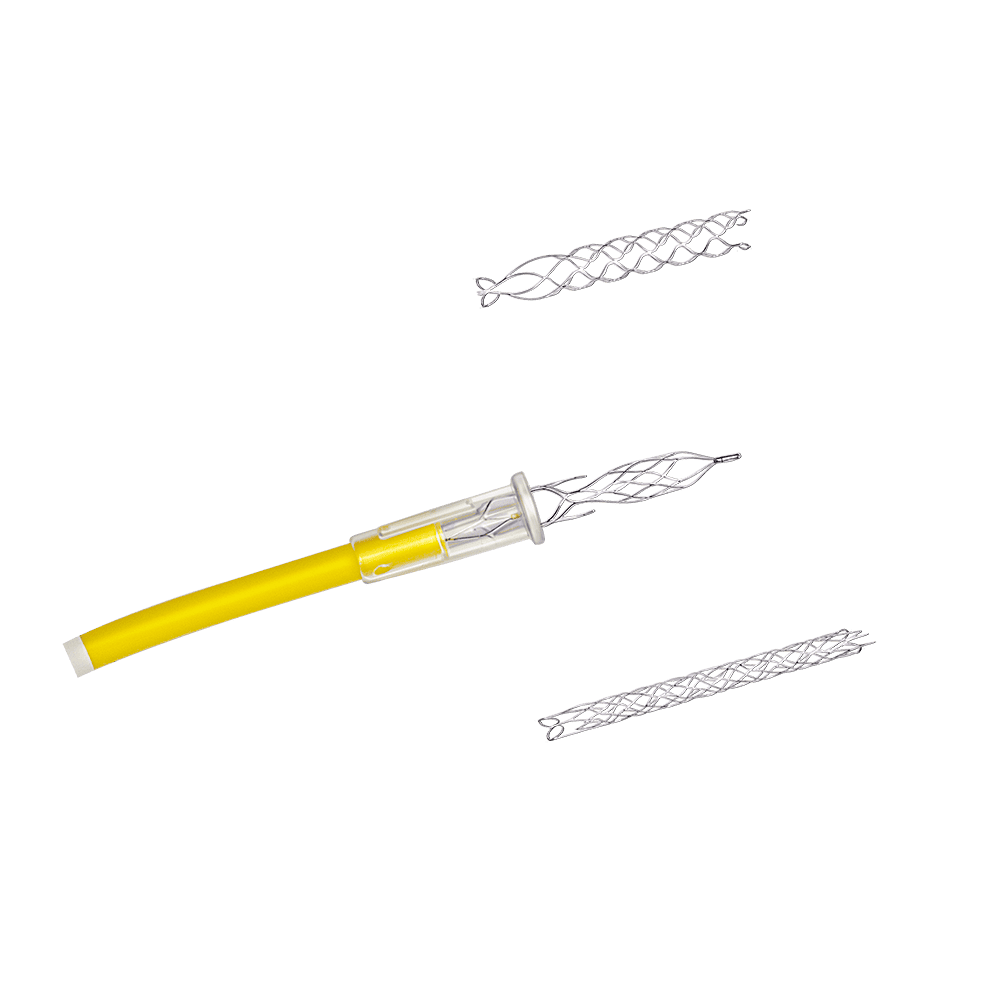
We provide product design teams with a capability never before achieved on a production-ready platform. The narrow kerf capability combined with accelerated processing speed makes STEALTH laser machining technology the solution for newest vascular implant designs with the smallest geometry and highest fatigue requirements.
We provide product design teams with a capability never before achieved on a production-ready platform. The narrow kerf capability combined with accelerated processing speed makes STEALTH laser machining technology the solution for newest vascular implant designs with the smallest geometry and highest fatigue requirements.