Nitinol is essential to many of today’s most advanced medical devices, and this FAQ highlights how Norman Noble supports OEMs with the manufacturing expertise needed to bring complex designs to life.
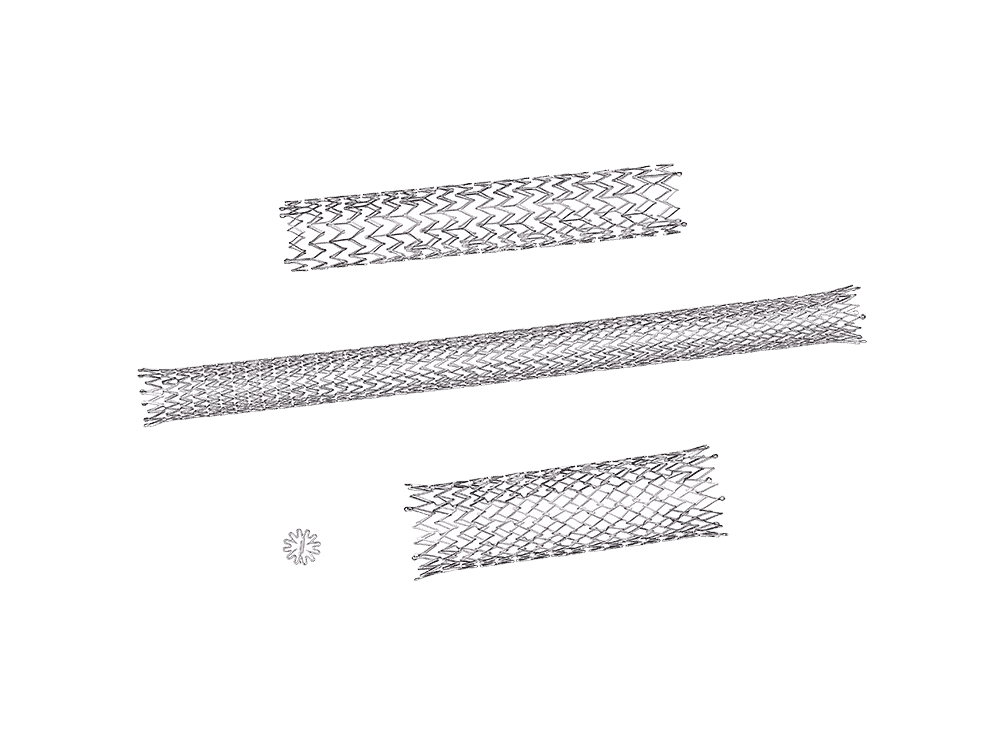
Nitinol is a nickel-titanium alloy known for its shape memory and superelasticity. These properties allow components to return to their original shape after deformation, making nitinol ideal for minimally invasive medical devices, including vascular implants, orthopedic tools, and robotic surgical components.
Norman Noble provides end-to-end nitinol manufacturing, including:
Nitinol laser machining for ultra-fine features
Nitinol shape-setting using proprietary fixturing and controlled thermal processes
Nitinol micro-machining for complex geometries
Nitinol electropolishing, surface finishing, and metrology
Full process validation and device-grade quality systems
Our vertically integrated processes allow OEMs to move from prototype to high-volume production with consistent control of dimensional tolerances and material performance.
Shape-setting involves placing Nitinol components into precision-engineered fixtures and applying controlled heat treatment cycles. This thermal processing “programs” the alloy to maintain a specific shape. Norman Noble utilizes advanced furnaces, custom tooling, and statistical process controls to ensure stable transformation temperatures and repeatable device performance.
Laser machining creates highly precise features in nitinol without compromising material properties. Benefits include:
Ultra-tight tolerances
Smooth cut edges ideal for implants
Ability to machine thin-wall tubing and complex stent-like geometries
Reduced need for secondary manual or mechanical processing
Compatibility with high-volume, automated production lines
Nitinol is frequently used in:
Vascular implants (stents, filters, clot retrieval devices)
Orthopedic implants and instruments
Yes. Our Process Development Centers support rapid iterations for R&D teams, while our production facilities deliver automated, high-volume manufacturing with validated processes. This seamless transition allows OEMs to shorten development timelines and reduce risk.
Quality is built into every step:
ISO 13485-certified and FDA-registered facilities
In-house CT scanning, microscopy, dimensional metrology, and materials analysis
Automated laser systems with real-time monitoring
Documented process controls and traceability
Engineering support for design for manufacturability (DFM) and risk mitigation
Absolutely. Our engineering team collaborates with OEMs early to optimize wall thickness, feature geometry, bend radii, transformation temperatures, and surface requirements. This reduces development cycles and improves long-term manufacturing scalability.
Exact tolerances depend on geometry and wall thickness, but our micro-laser machining platforms routinely achieve micron-level accuracies. For highly complex nitinol components, we combine laser machining, finishing, and metrology to achieve high quality product within tight tolerances.
We support OEMs with:
IQ/OQ/PQ validation
Material certifications
Lot traceability
Process documentation and risk analyses
Support for FDA and other OUS regulatory submissions and inspections
Our commitment to controlled manufacturing helps OEM engineers meet stringent regulatory expectations.
OEMs choose Norman Noble for:
Decades of experience in precision laser machining and nitinol processing
Proprietary shape-setting and high-speed stent machining systems
Fully integrated manufacturing—from prototype to production
A culture of engineering collaboration and rapid innovation
Industry-leading investment in inspection technology, automation, and quality systems
Simply reach out through our website’s Contact or Request a Quote form. Our engineering and applications teams will review your device design, discuss manufacturability options, and propose the best path for prototyping, validation, and production.