Our engineers design custom fixturing and validated processes to produce next-gen nitinol medical devices and implants.
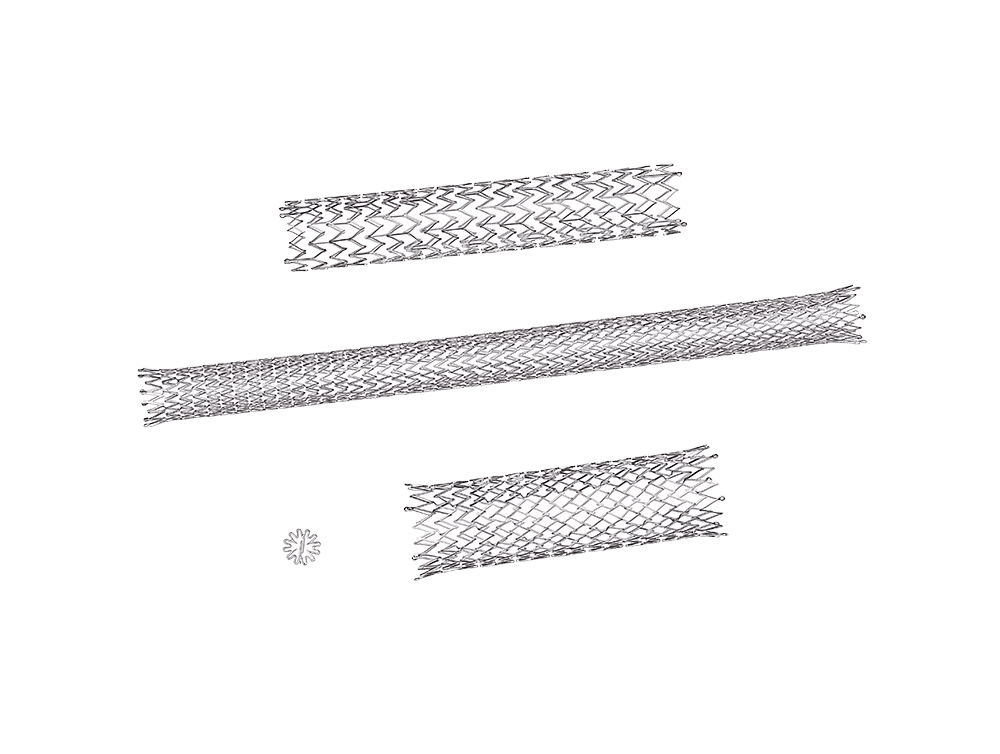
The nickel and titanium alloy known as Nitinol, is a superelastic shape-memory alloy responsible for major advances in medical technology over the last 25 years.
The demand for smaller devices and entirely new neurovascular applications requires even more innovative manufacturing methods.
Extensive athermal laser machining capabilities and expertise allow us to test and produce next-generation implants.
Norman Noble’s proprietary S.T.E.A.L.T.H. Athermal Laser Technology is capable of manufacturing parts with kerf widths of .0004” with no heat-affected zone.
At a minimum, Norman Noble qualifies and validates two sources of Nitinol for each production device or implant.
The quality, type (sheet or tube), and characteristics of the Nitinol supplied by any given raw material producer is variable. Since Norman Noble regularly sources material from many suppliers, we are able to match the raw material to custom design requirements and product applications.
Norman Noble engineers evaluate nitinol product design specifications during the prototyping stage to identify opportunities to reduce cost without compromising the manufactured part’s intended function.
For any prospective new device design, extensive design and testing services are available to help the design engineer perform FEA.
This ability to model and simulate mechanical behavior reduces the time needed between design iterations, a critical step in the race to bring products to market.
At Norman Noble, we custom design each manufacturing step for any particular nitinol product design.
This is done by designing, testing, and refining each step using the same model and type of equipment and conditions that will be used in production.
The capability to design and manufacture all shape-set tooling and fixturing for each process step in-house is essential to ensure the highest level of quality and process control.
Norman Noble does this testing using equipment, experienced engineering personnel, and facilities dedicated solely to process development, so the development of next-gen devices do not compete for time with devices already in the production-manufacturing stream.
Producing finished Nitinol parts is a complex, multi-phase endeavor requiring years of experience and numerous manufacturing and finishing process capabilities.
At Norman Noble, we completely manufacture your part in-house using special Nitinol processing techniques.
By handling all steps in the manufacturing process, we can tune each step based on our knowledge of the upstream or downstream capabilities. This produces the highest level of quality assurance and process control.
Closely regulated, Class 3 medical implants and devices manufactured to the highest specification are life-saving devices.
One of the last steps in producing a device, dimensional inspection, is also one of the most critical to verifying a consistent, high-quality component that meets design specifications.
Norman Noble has the capability to provide validated, 100% dimensional inspection on each and every implant.
All operational process steps and tooling used to manufacture Nitinol implants must be within established and tested control limits.
Validation ensures that the manufacturing process conforms to the control limits.
Inspection ensures the manufacturing output does as well.
Validation must be conducted in accordance to ISO 13485:2016 standards.
Norman Noble has an experienced validation engineering team to provide the strategy and protocols needed to complete all validation activities.