Rapid prototype through production.
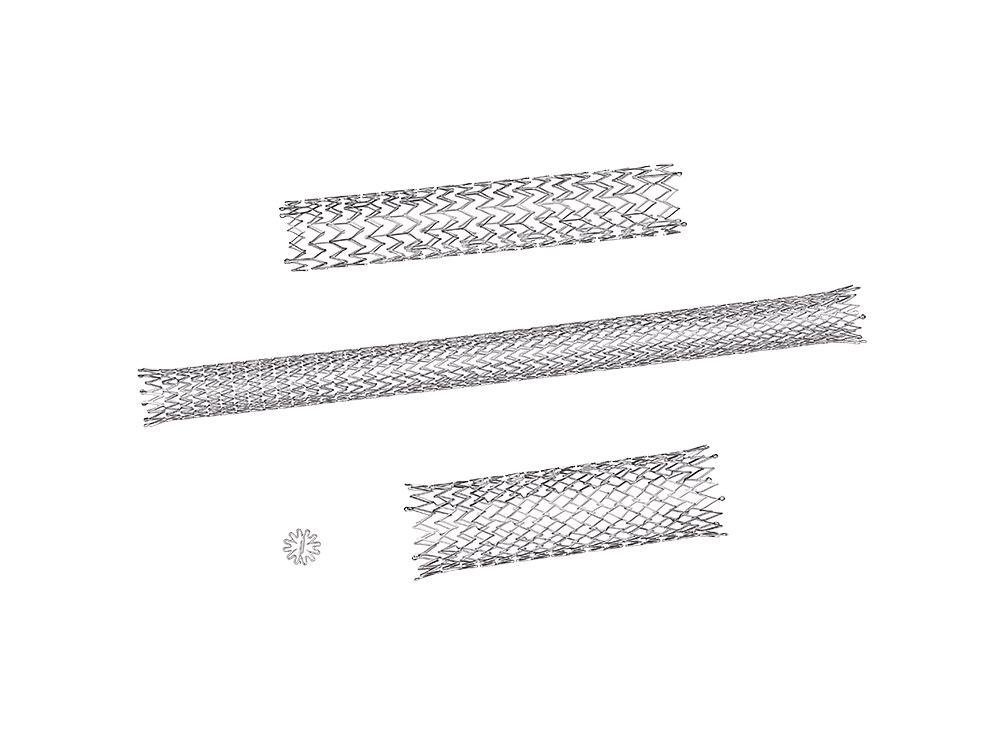
At Norman Noble, our nitinol stent machining, heart valve machining and clot puller machining technologies are second to none. We utilize dedicated process development engineering teams to design custom manufacturing and nitinol electropolish finishing processes for the most challenging of next-gen vascular implants and nitinol medical devices and implants.
With more than 125 proprietary laser machines, shape setting nitinol, automated nitinol electropolishing and dimensional inspection systems, Norman Noble supports vascular implant manufacturing from prototype through production. Our vertically integrated stent manufacturing and finishing technologies produce active implants from exotic materials that have the most unique geometries and tightest tolerances.
At Norman Noble, our nitinol stent machining, heart valve machining and clot puller machining technologies are second to none. We utilize dedicated process development engineering teams to design custom manufacturing and nitinol electropolish finishing processes for the most challenging of next-gen vascular implants and nitinol medical devices and implants.
With more than 125 proprietary laser machines, shape setting nitinol, automated nitinol electropolishing and dimensional inspection systems, Norman Noble supports vascular implant manufacturing from prototype through production. Our vertically integrated stent manufacturing and finishing technologies produce active implants from exotic materials that have the most unique geometries and tightest tolerances.

Hover over parts to expand images.
Click parts to expand images.